Abstract
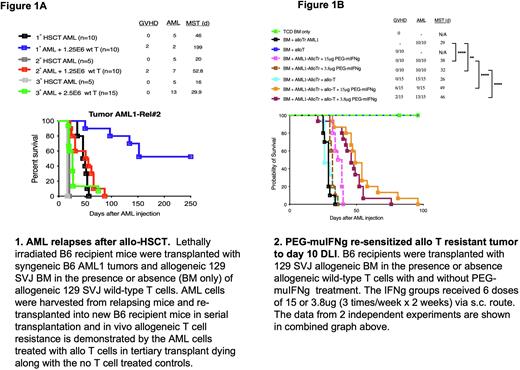
Background: The prognosis of AML relapsing after allogeneic hematopoietic stem cell transplant (alloHSCT) is dismal with very few salvage therapy options. Knowing the biological changes that occur at relapse could lead to potential treatment strategies. Using murine AML cells (Dnmt3aR878H/FLT3-ITD model, kind gift from Dr. Ley's lab), we generated allogeneic T cell resistant murine AML cells (alloTr AML1) via sequential MHC-mismatched alloHSCTs. As shown in Figure 1A, mice injected with the AML1 tumor and allogeneic T cells displayed a significantly decreased median survival time (MST) from >250 days (undefined) to 26 days during progression from the primary alloHSCT to the tertiary alloHSCT, respectively (P<0.0001, 1° HSCT vs. 3° HSCT). Genomic analysis using WES showed no mutations in MHC I, MHC II or genes associated in immune functions in the alloTr AML1 cells. Preliminary transcriptome analysis of the alloTr AML1 after the tertiary transplant shows downregulation of genes mediating cell-cycle regulation and activation of T cells, including regulation of IFN-gamma (IFNg) production pathways. Our group previously showed that human AML cells relapsing after alloHSCT downregulated MHC class II and that IFNg could re-induce MHC-II expression and restore allogeneic T cell-mediated cytotoxicity (Christopher et al, NEJM, 2019). Since native IFNg has a very short terminal half-life of approximately 1h in vivo, it necessitates frequent administration to maintain sustained therapeutic levels. Our collaborators at Bolder Biotech have generated site-specific PEGylated IFNg that exhibits a terminal half-life of 26h in rats and can be detected in the plasma for at least 6 days after a single injection (Fam et al, J Interferon & Cytokine Research, 2014). Here, we evaluated if PEGylated murine (PEG-muIFNg) from Bolder Biotech could re-sensitize alloTr AML1 cells to T cell killing after allo-HSCT.
Method: Lethally irradiated B6 recipients were reconstituted with 129 SVJ bone marrow (BM) alone on day 0. On day 10 after alloHSCT, mice were injected with 1.5E6 alloTr AML cells and divided into the following groups: (1) untreated controls (BM only), (2) PEG-muIFNg only, (3) 5 x 106 allogeneic 129 SVJ T cells (allo-T) only on day 10 or (4) 5 x 106allogeneic 129 SVJ T cells on day 10 plus PEG-muIFNg. Two doses of PEG-muIFNg (15 ug or 3.8 ug per injection) were tested in these experiments and treatment was started on day 10 post-alloHSCT at the time of allo-T injection. A total of 6 PEG-muIFNg doses were given (3 doses/week x 2 weeks). Mice were monitored for survival and graft-versus-host disease (GVHD). AML1 tumor burden was assessed by FACS for GFP.
Results: The untreated BM only controls died of AML within 18 days of tumor injection (Figure 1B). Treatment with PEG-muIFNg alone, slightly extended the median survival time (MST) of mice in a dose dependent manner with 15 ug PEG-muIFNg dosing providing a MST of 38 days while the 3.8ug dose level exhibited a MST of 32 days. Recipients treated with 5 x 106 allo-T cells alone succumbed to leukemia similar to the untreated controls (MST = 26 days), confirming that these cells were resistant to allo-T cell killing. However, mice treated with allo-T + 15 ug PEG-muIFNgexhibited prolonged survival (MST = 49 days) compared to the T cell only (MST= 26 days; p=0.0001) and the 15 ug PEG-muIFNg only controls (MST =38 days; p=0.0001). The 15 ug dose level provided greater graft-versus-leukemia (GVL) activity (only 9/15 mice relapsed vs. 13/15 with 3.8 ug dosing) but was associated with slightly higher GVHD (6/15 mice developed lethal GVHD). In contrast, the 3.8 ug dose had fewer GVHD related deaths (2/15) but provided slightly less GVL activity (13/15 mice succumbed to AML).
Conclusion: Treatment with PEG-muIFNg beginning 10 days after allo-HSCT can re-sensitize the alloTr AML1 tumors to allogeneic T cell-mediated killing and provide a significant survival advantage. Ongoing studies are also examining the candidate IFNg signaling pathway genes dysregulated in our model to determine if they are upregulated after PEG-muIFNg treatment. Furthermore, future studies will explore if extending PEG-muIFNgtreatment at the 3.8 ug concentration beyond 2 weeks can enhance GVL activity while limiting lethal GVHD.
Disclosures
Cox:Bolder BioTechnology: Current Employment, Current equity holder in private company, Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees, Patents & Royalties, Research Funding. Anderson:Bolder BioTechnology: Current Employment, Current holder of stock options in a privately-held company. DiPersio:VLA-4 Inhibitor with Washington University and Magenta Therapeutics: Patents & Royalties; Amphivena Therapeutics: Research Funding; Incyte: Consultancy, Research Funding; RiverVest Venture Partners: Consultancy, Membership on an entity's Board of Directors or advisory committees; BioLineRx, Ltd.: Research Funding; hC Bioscience, Inc.: Membership on an entity's Board of Directors or advisory committees; Macrogenics: Research Funding; NeoImmune Tech: Research Funding; CAR-T cell Product with Washington University and WUGEN: Patents & Royalties; WUGEN: Current equity holder in private company, Research Funding; Magenta Therapeutics: Current equity holder in private company, Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal